How Does This Affect The Reaction Rate Along Each Pathway: Insights Unveiled
Gcse Chemistry – Factors Affecting The Rate Of Reaction #47
Keywords searched by users: How does this affect the reaction rate along each pathway how does catalyst affect the rate of chemical reaction, how does catalyst affect the rate of reaction collision theory, What is a catalyst and a catalysis reaction, how does a catalyst affect activation energy of a chemical reaction, how does surface area affect the rate of chemical reaction, How does temperature affect the reaction rate, how does temperature affect reaction rate, how does concentration affect reaction rate
How Does It Affect Rate Of Reaction?
The rate of a chemical reaction can be influenced by several key factors. Firstly, increasing the concentration of a reactant in a solution tends to accelerate the reaction. This is because a higher concentration means there are more reactant particles available to collide and interact with each other. Additionally, when dealing with solid reactants, breaking them into smaller pieces to increase their surface area can also enhance the reaction rate. This provides more contact points for reactant particles, promoting faster collisions. Furthermore, elevating the temperature of the reaction system is known to boost reaction rates. Higher temperatures provide reactant particles with increased kinetic energy, causing them to move more vigorously and collide more frequently, leading to faster reactions.
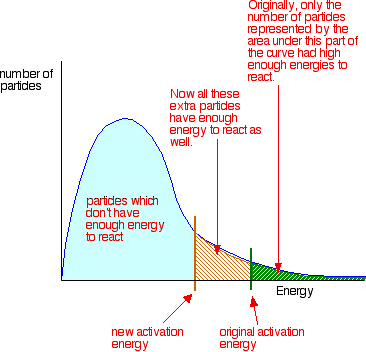
Moreover, another way to expedite a reaction is by introducing a catalyst into the reaction mixture. Catalysts are substances that facilitate reactions by lowering the activation energy required for the reaction to occur, making it easier for reactants to transform into products. By promoting these factors, the rate of a reaction can be significantly enhanced, allowing for more efficient chemical processes. This information can be applied to various chemical reactions, aiding researchers and scientists in optimizing reaction conditions for desired outcomes.
How Does Collision Theory Affect Rate Of Reaction?
The concept of collision theory plays a crucial role in influencing the rate of chemical reactions. According to this theory, the rate at which a chemical reaction occurs is directly related to the frequency of collisions between the molecules of the reactants involved. In other words, the more frequently these reactant molecules collide, the greater the likelihood that they will interact and undergo chemical transformations. Consequently, a higher collision frequency leads to a faster reaction rate. This fundamental principle underscores the importance of molecular collisions in determining the speed at which chemical reactions take place.
How Does The Rate Of A Reaction Change As The Reaction Progresses?
How does the rate of a chemical reaction evolve over time? In most cases, as a reaction unfolds, its rate tends to diminish. This phenomenon occurs due to the declining concentrations of the reactants, which are gradually transformed into products as the reaction proceeds. Conversely, it’s important to note that reaction rates often exhibit an upward trend when the concentrations of the initial reactants are augmented. To illustrate, let’s consider an example: on July 12th, 2023, this principle was demonstrated in a specific chemical reaction.
Summary 41 How does this affect the reaction rate along each pathway


:max_bytes(150000):strip_icc()/catalystenergydiagram-56a12b265f9b58b7d0bcb2fe.jpg)



Categories: Found 99 How Does This Affect The Reaction Rate Along Each Pathway
See more here: future-user.com

Learn more about the topic How does this affect the reaction rate along each pathway.
- Catalyst | CK-12 Foundation
- Factors affecting reaction rates (video) – Khan Academy
- Kinetics and Collision Theory – Harper College
- 14.3: Effect of Concentration on Reaction Rates: The Rate Law
- How does activation energy is related to the rate of reaction? – Toppr
- Reaction Times
See more: https://future-user.com/your-money blog